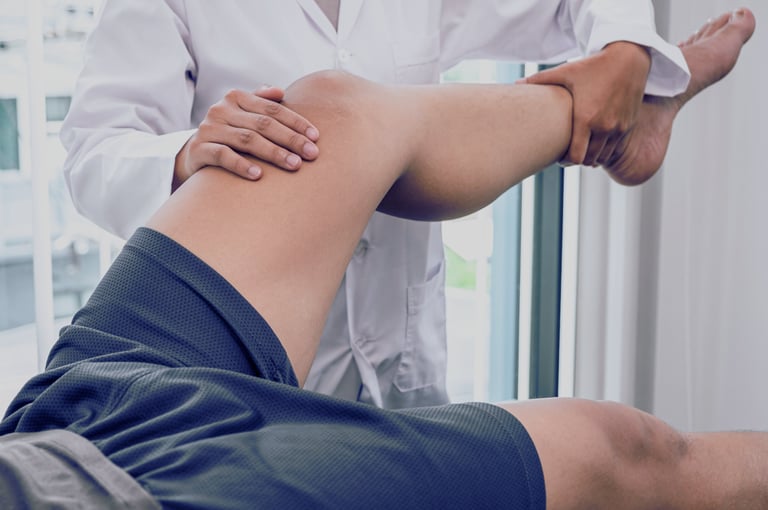
Your own cells for knee cartilage regeneration
Introducing RECARTA
RECARTA is designed to regenerate cartilage utilizing patients' autologous cells - chondrocytes


RECARTA is produced with adherence to quality Good Manufacturing Practice (GMP) standards, using patient's own cells in a sterile clean room with automated environmental controls and monitoring.
The manufacturing process is closely monitored and tested for microbial contamination. Highly qualified personel is ensuring the safety and consistency in preparation process. Trained scientists and engineers involved wearing pre-sterilized protective gear to assure the safe production of RECARTA.
Implemented strict quality control checks throughout the process and at the release for implantation are in place to monitor and secure the quality and the safety of the process.
Prepared in accordance with GMP standards
STEP ONE
Consultation


Symptoms, such as knee pain, swelling, crackling, and locking, frequently interfere with individuals' preferred activities and can be a cause of discomfort.
Your orthopedic surgeon can advise you on the further appropriate actions. X-ray and Magnetic Resonance Imaging will show the extent of your cartilage damage. After consultation, your doctor will assess if you are eligible for RECARTA or other treatment options.
RECARTA involves the expansion of autologous chondrocytes, which are then placed onto a membrane and implanted into the affected region of the cartilage.
How RECARTA works?
STEP TWO
Harvesting your cells and Manufacturing
A short, minimally invasive arthroscopic surgical procedure is to harvest a ballpen-size cartilage biopsy sample from a non-weight-bearing area of your damaged knee. You leave home the same day after the procedure.
The cartilage biopsy sample is placed in a special nutrient-rich media and secured in a temperature-controlled transport carrier. The carrier is transferred to the accredited clean-room laboratory facility, where the preparation process takes up to 4 weeks.
Specialized, natural cartilage-forming cells are isolated and multiplied into millions of cells through an advanced biotechnological and strict quality-controlled approach.
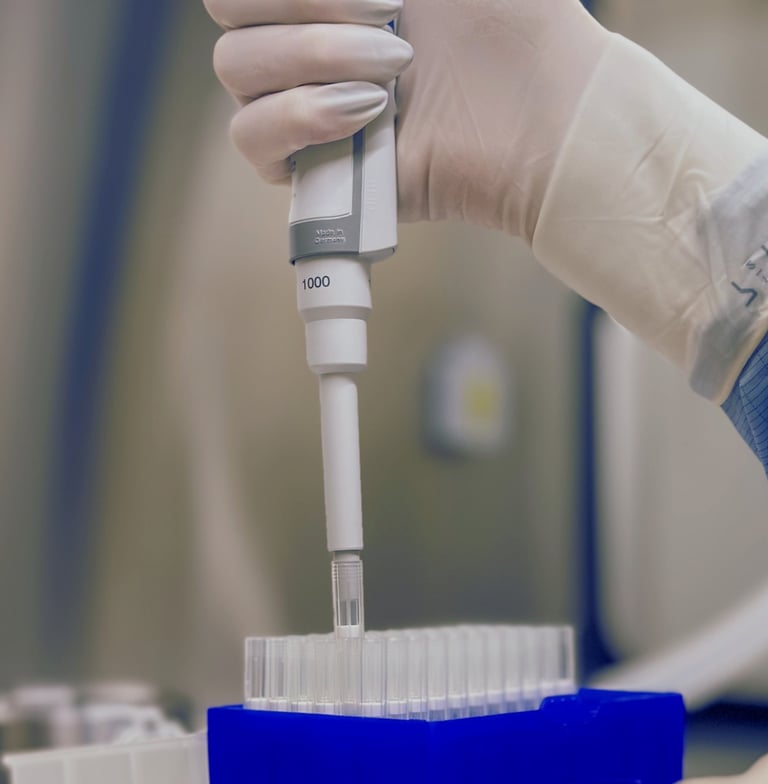
STEP THREE
Implantation
RECARTA is rigorously evaluated for safety and efficacy throughout and at the end of the preparation process. After confirmation of passing the quality control tests, RECARTA is released for implantation.
Cartilage-forming cells are combined with a biological scaffold for improved attachment and proliferation. The tissue-engineered product is introduced back to the defect, and the cartilage regeneration has started.
STEP FOUR
Rehabilitation
A cartilage regeneration process is enforced with a personalized rehabilitation stage. You will be provided a customized concierge-type rehabilitation therapy after RECARTA implantation.
Extensive follow-up will ensure your cartilage is undergoing a durable regeneration. A thorough rehabilitation will safeguard the newly formed cartilage for the upcoming decades to support a life-long healthy knee functioning.