
Our Pipeline
Building a pipeline for advanced therapy medicines
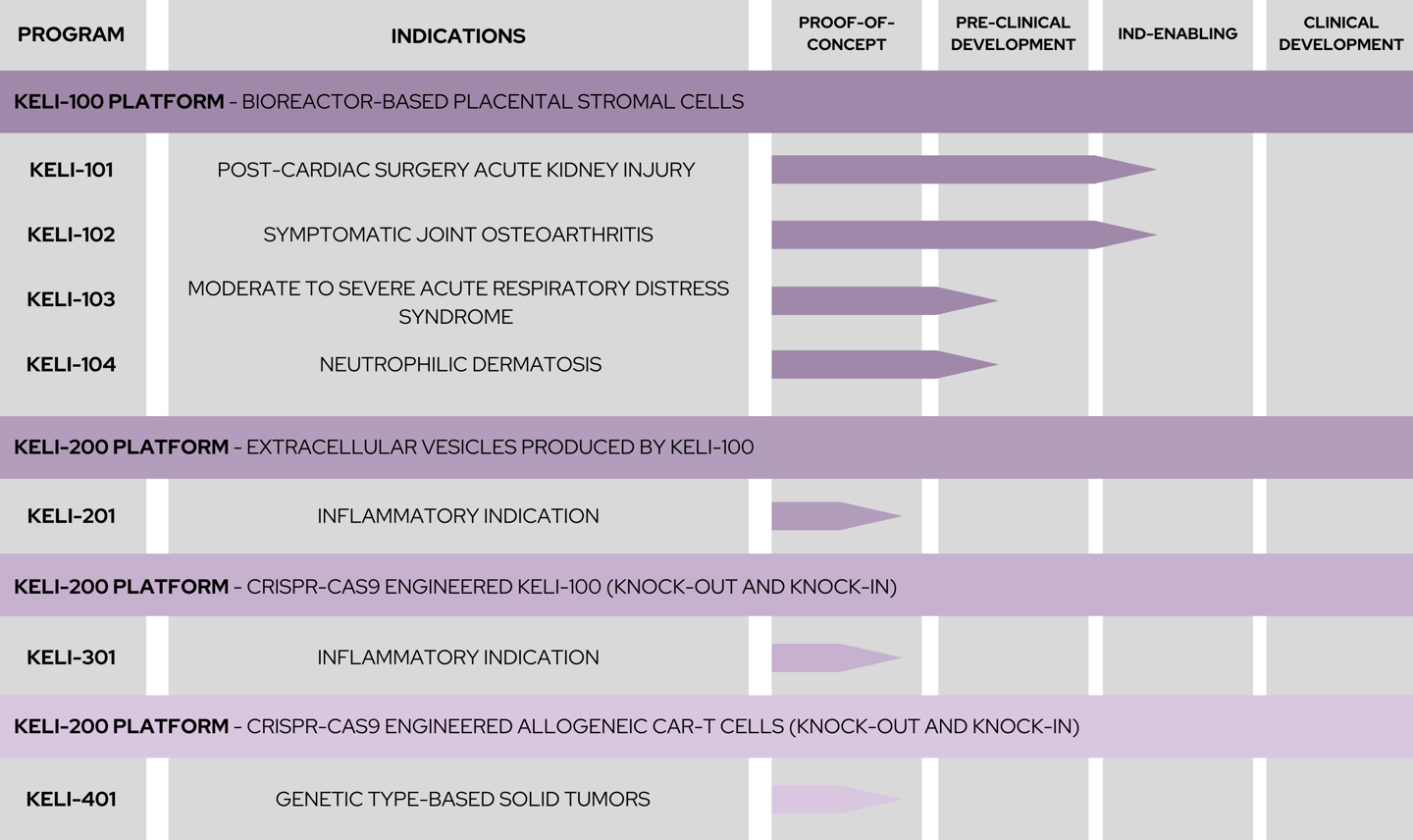
KELI Therapeutics' pipeline consists of programs at different stages of development within four platforms. We target quantifiable medical benefits in multiple inflammatory and oncology therapeutic areas
PLATFORM
PROGRAM
INDICATIONS
PRE-CLINICAL
IND-ENABLING
CLINICAL DEVELOPMENT
KELI-101
KELI-102
KELI-103
KELI-104
Post-cardiac surgery acute kidney injury
Moderate to severe acute respiratory distress syndrome
Neutrophilic dermatosis
KELI-100
KELI-200
KELI-201
Undisclosed chronic inflammatory and fibrotic pathogenesis
KELI-300
KELI-301
Symptomatic joint osteoarthritis
Undisclosed
Information is current as of 5/08/2025, updates are made every six months
KELI-100
KELI-200
KELI-300
Bioreactor-based placental stromal cells
CRISPR-Cas9 engineered KELI-100 (knock-out and knock-in)
Extracellular vesicles produced by KELI-100
Information is current as of 9/23/2023, updates are made on quarterly basis
PLATFORM
PROGRAM
INDICATIONS
PROOF-OF-CONCEPT
PRE-CLINICAL
IND-ENABLING
CLINICAL DEVELOPMENT
KELI-101
KELI-102
KELI-103
KELI-104
Post-cardiac surgery acute kidney injury
Moderate to severe acute respiratory distress syndrome
Neutrophilic dermatosis
KELI-100
KELI-200
KELI-201
Inflammatory indication
KELI-300
KELI-301
Symptomatic joint osteoarthritis
KELI-400
KELI-401
Inflammatory indication
Genetic type-based solid tumors
Information is current as of 9/23/2023, updates are made on quarterly basis
Human placenta stromal cells
Extracellular vesicles produced by KELI-100
CRISPR-Cas9 engineered KELI-100 (knock-out and knock-in)
CRISPR-Cas9 engineered allogeneic CAR-T cells (knock-out and knock-in)
PROOF-OF-CONCEPT
PRE-CLINICAL
IND-ENABLING
CLINICAL DEVELOPMENT
KELI-100 Platform - bioreactor-based placental stromal cells
SYMPTOMATIC JOINT OSTEOARTHRITIS
POST-CARDIAC SURGERY ACUTE KIDNEY INJURY
MODERATE TO SEVERE ACUTE RESPIRATORY DISTRESS SYNDROME
NEUTROPHILIC DERMATOSIS
KELI-200 Platform - extracellular vesicles produced by KELI-100
PROOF-OF-CONCEPT
PRE-CLINICAL
IND-ENABLING
CLINICAL DEVELOPMENT
INFLAMMATORY INDICATIONS
KELI-300 Platform - CRIPR-Cas9 engineered KELI-100 (knock-out and knock-in)
PROOF-OF-CONCEPT
PRE-CLINICAL
IND-ENABLING
CLINICAL DEVELOPMENT
INFLAMMATORY INDICATIONS
KELI-101
KELI-102
KELI-103
KELI-104
KELI-201
KELI-301
KELI-400 Platform - CRISPR-CAS9 engineered allogeneic CAR-NK cell (knock-out and knock-in)
CLINICAL DEVELOPMENT
PROOF-OF-CONCEPT
PRE-CLINICAL
IND-ENABLING
GENETIC TYPE-BASED SOLID TUMORS
KELI-401
Information is current as of 9/23/2023, updates are made every six months
PROGRAM
POST-CARDIAC SURGERY ACUTE KIDNEY INJURY
CLINICAL DEVELOPMENT
PHASE
KELI-100 Platform - human placenta stromal cell
SYMTPMATIC JOINT OSTEOARTHRITIS
MODERATE TO SEVERE ACUTE RESPIRATORY DISTRESS SYNDROME
NEUTROPHILIC DERMATOSIS
KELI-200 Platform - CRIPR-Cas9 engineered KELI-100 (knock-out and knock-in)
KELI-300 Platform - extracellular vesicles produced by KELI-100
KELI-102
KELI-103
KELI-104
KELI-201
Information is current as of 5/08/2025, updates are made every six months
IND-ENABLING
KELI-101
INDICATION
IND-ENABLING
IND-ENABLING
PROGRAM
INDICATION
PHASE
UNDISCLOSED CHRONIC INFLAMMATORY AND FIBROTIC PATHOGENESIS
PRE-CLINICAL
PROGRAM
UNDISCLOSED
INDICATION
PHASE
KELI-301
PRE-CLINICAL
