
Research Products
A range of products that are essential tools designed for the development and testing of therapeutics
CELLS
EXTRACELLULAR VESICLES
CELL CULTURE MEDIA KITS
We manufacture extensively characterized GMP-like Cells for research, non-clinical applications
We provide readily available Extracellular Vesicles derived from placental mesenchymal stromal cells, ready for use in non-clinical applications
Our Cell Culture Media Kits contain basal media with supplement and are adapted to support the growth of Cells
ELEVATING RESEARCH TO REVOLUTIONARY CELL THERAPY
Our portfolio includes GMP-compliant and research-grade mesenchymal stromal (MSCs) and somatic (chondrocyte) cells suitable for preclinical research.
These cells have been produced with exceptional quality and efficacy parameters and tested in many preclinical studies.


FROM BENCH TO BREAKTHROUGHS


hPMSCs (subtypes)
QC Information
Type options: All subtypes
Culture options: FBS/ hPL
Culture conditions options: 2D/ 3D
Passage options: P0/ P1/ Custom
Shipping options: Cryopreserved/ Proliferating
Quantity options: 1M cells/vial / Custom
Quality options: GMP-like/ GMP
Human Placenta-derived Mesenchymal Stromal Cells (hPMSCs) exhibit the characteristic markers and functions of MSCs. As a result, they serve as a valuable model for investigating differentiation, inflammation, tissue homeostasis, and regeneration.
The placenta is a complex tissue formed from contributions from both maternal and fetal tissues, leading to a higher diversity of cell types.
KELI manufactures specialized subtypes of placental cells options: Placental Chorionic villi MSCs; Placental Decidua basalis MSCs; Placental Amniotic epithelial cells; Placental Amniotic MSCs; Chorionic plate trophoblast cells; Chorionic plate MSCs; Decidua NK-CD56+CD3-; Umbilical cord (whole) MSCs; Umbilical cord tissue MSCs; Umbilical cord Wharton jelly MSCs.
Culture conditions/
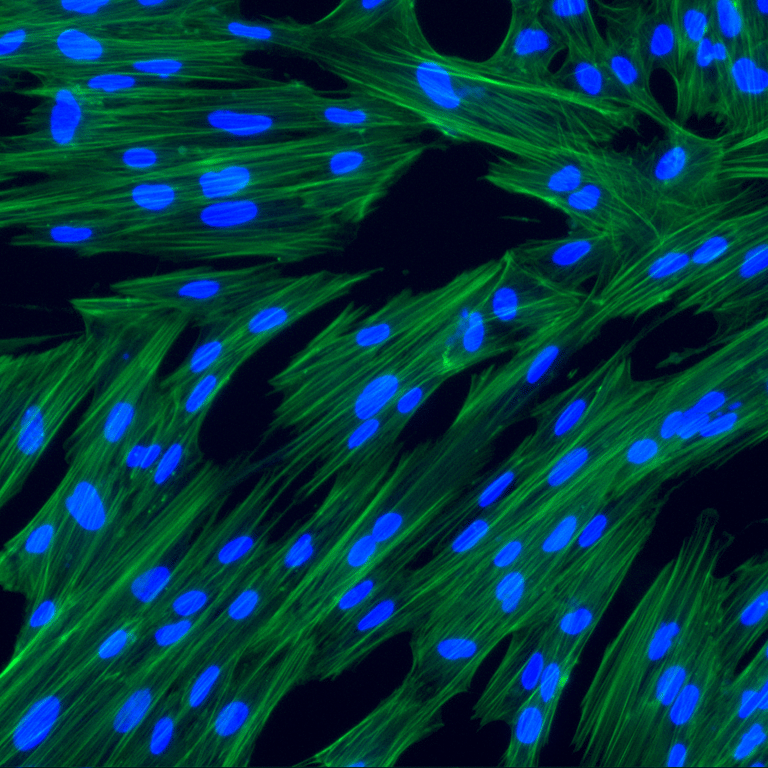
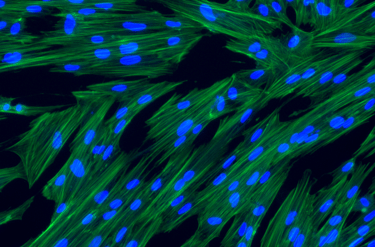
hPMSCs are qualified and tested for safety and quality per European Pharmacopeia
Placenta donor tests: HIV 1/2, HBsAg, anti-HBcor, anti-HCV, anti-HBs, and T. pallidum
Safety tests on hPMSCs: sterility, mycoplasma, and endotoxin
Phenotypic identity: FACS analysis
Potency evaluation: lymphocyte proliferation and anti-inflammatory assays
Stability and storage: liquid nitrogen maintains cell viability and functionality for no less than four years.
Shipping: worldwide delivery of cells and EVs
Mesenchymal Stromal Cells
CELLS
KELI has developed and produced cellular products for research organizations and internal non-clinical and clinical studies. This involvement includes handling tissue acquisition, cell upstream and downstream processes, large-scale bioreactor cultures, and quality control analysis. Our specialized knowledge allows us to optimize the conditions for different types of cells, ensuring consistent cellular quality. We are adept at establishing robust manufacturing processes and meeting stringent regulatory requirements.
Chondrocytes
Chondrocytes derived from humans serve as valuable in vitro models for investigating cartilage regeneration and repair, exploring the impacts of cytokines and growth factors on cartilage, examining specific gene regulation, and unraveling arthritis pathophysiology. KELI Therapeutics offers human chondrocytes isolated from healthy articular cartilage (hCHs) and osteoarthritic cartilage (hOACHs) from the knee of individual donors.
Product Information
hCHs and hOACHs are qualified and tested for safety and quality per European Pharmacopeia
Cartilage donor tests: HIV 1/2, HBsAg, anti-HBcor, anti-HCV, anti-HBs, and T. pallidum
Safety tests on hCH: sterility, mycoplasma, and endotoxin
Potency evaluation: COL2A1 expression analysis
Stability and storage: liquid nitrogen maintains cell viability and functionality for no less than four years.
Shipping: worldwide delivery of cells on dry ice
Downloads
Certificate of analysis (CoA) is available with order
(#CH-01) coming soon...
(#CH-01) coming soon...
#PSC-01 (example)
#PSC-01 (example)
Certificate of analysis (CoA) is available with order
hCHs, cryopreserved
Cat#: CH-01
Passage: One
Cells/vial: 500,000
hOACHs, cryopreserved
Cat#: CH-02
Passage: One
Cells/vial: 500,000
Safety Data Sheet (customised to the cell/ EVs subtype):
Product Data Sheet (customised to the cell/ EVs subtype):
(#CH-02) coming soon...
(#CH-02) coming soon...
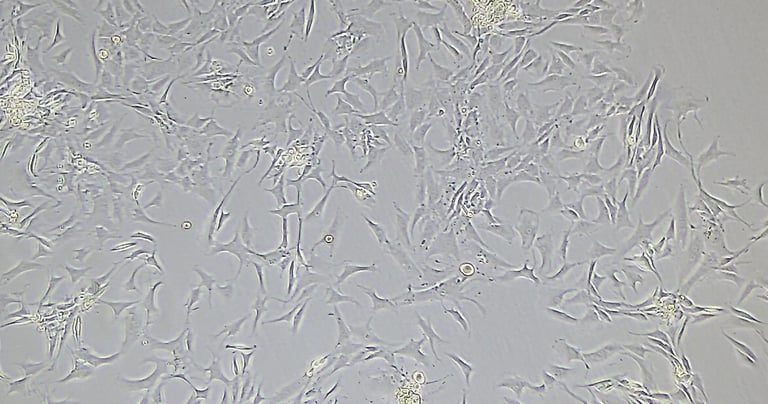
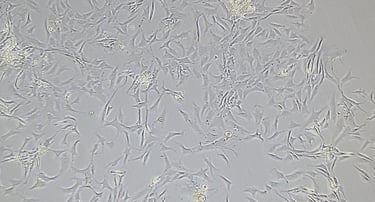
Mesenchymal Stromal Cells Media Kit
The cell culture system supports in vitro expansion and differentiation of hPMSCs. A kit includes a Basal Medium and Supplement for a Complete Culture Medium formulation. Two versions are available: Basal media with serum-containing supplement (PL-CM) and basal media with exosomes-free supplement (PL-CM-EF).
Product Information
Kit Components:
Basal Media (PL-BM), 500 mL
Supplement (PL-SP), 50 mL


PL-CM
Cat#: KIT-01
PL-CM-EF
Cat#: KIT-02
Rigorous quality control tests ensure reliable and consistent product quality.
Safety tests on media lots: sterility, mycoplasma, and endotoxin
Growth promotion: supports the growth of GMP-grade hPMSCs and EVs collection
Shipping: worldwide delivery of media and supplement
Kit Components:
Basal Media (PL-BM), 500 mL
Supplement (PL-SP-EF), 50 mL
CELL CULTURE MEDIA KITS
KELI Therapeutics offers cell culture media and reagents for culturing primary cells — specialized cell culture media kits for human Placental Mesenchymal Stromal Cells and Chondrocytes.
Chondrocytes Media Kit
Chondrocytes Media Kit (CH-CM) is a complete medium designed for optimal growth of primary chondrocytes in vitro.
Product Information
Rigorous quality control tests ensure reliable and consistent product quality.
Safety tests on media lots: sterility, mycoplasma, and endotoxin
Growth promotion: supports the growth of GMP-grade human chondrocytes
Shipping: worldwide delivery of media and supplement
Downloads
(#KIT-03) coming soon...
(#KIT-03) coming soon...
Certificate of analysis (CoA) is available with order
Downloads
(#KIT-02) coming soon...
(#KIT-02) coming soon...
Certificate of analysis (CoA) is available with order
CH-CM
Cat#: KIT-03
Kit Components:
Basal Media (CH-BM), 500 mL
Supplement (CH-SP), 50 mL


EXTRACELLULAR VESICLES
Extracellular Vesicles (EVs) are collected and formulated from hPMSCs.
EVs play an essential role in cellular communication by transporting proteins, lipids, miRNAs, and mRNAs between cells. #EV-01 inhibits immune cells and contains molecules, reducing inflammation, and fibrosis and promoting tissue repair.
hPMSC EVs
Product Information
Downloads
Certificate of analysis (CoA) is available with order
10^9 EVs/100 µl, sterile


Cat#: EV-01
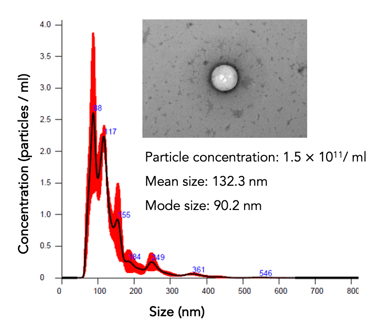
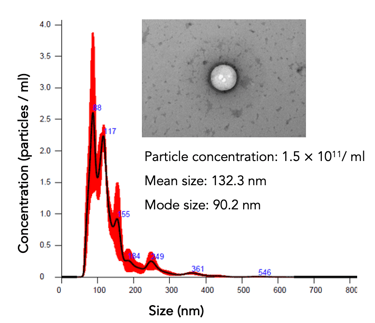
hPMSCs (a starting material for #EV-01) are qualified and tested for safety and quality per European Pharmacopeia
The method of preparation: tangential flow filtration
Safety tests: sterility, mycoplasma, and endotoxin
Phenotypic identity: FACS analysis
Potency evaluation: particle count, protein, and RNA concentrations. The cells' potency is assessed through various assays
Stability and storage: -80°C in the proprietary solution ensures EVs functionality for at least six months
